FDA Allows PM to Market IQOS as 'Reduced Risk' - Tobacco Asia
4.9 (138) · € 22.50 · Auf Lager

Why FDA's reduced exposure marketing order for IQOS is not a reliable global model – Stanton Glantz blog

FDA clears Philip Morris' iQOS, Altria prepares to sell the heated tobacco device in the US

US International Trade Commission bans imports of PMI's Iqos HeatSticks

Heated Tobacco Products - TobaccoTactics

10 facts about the FDA's modified risk tobacco product authorization of IQOS
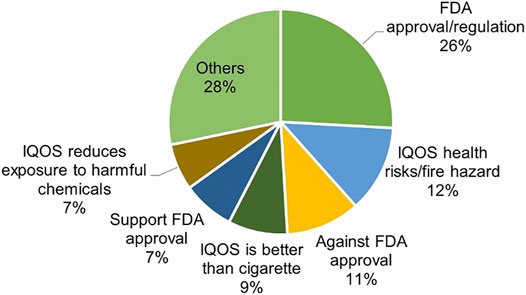
Frontiers Perceptions of the IQOS Heated Tobacco Product on Twitter in the United States

FDA Clears Philip Morris International Heat-Not-Burn IQOS Device for Sale in U.S. - WSJ

Tobacco giant Philip Morris sees future without cigarettes - Los Angeles Times

Heated Tobacco Products - TobaccoTactics

FDA Says PM USA Can Market IQOS as Modified Risk Tobacco Products
U.S. panel deals blow to Philip Morris tobacco device

Use of health warning content and FDA MRTP in Israel IQOS ad

Philip Morris develops zero-tobacco heat stick that may avoid regulations

SciELO - Saúde Pública - United States Food and Drug Administration's authorization of reduced exposure claims for IQOS®: implications for regulation in Latin America United States Food and Drug Administration's authorization of

FDA Says PM USA Can Market IQOS as Modified Risk Tobacco Products