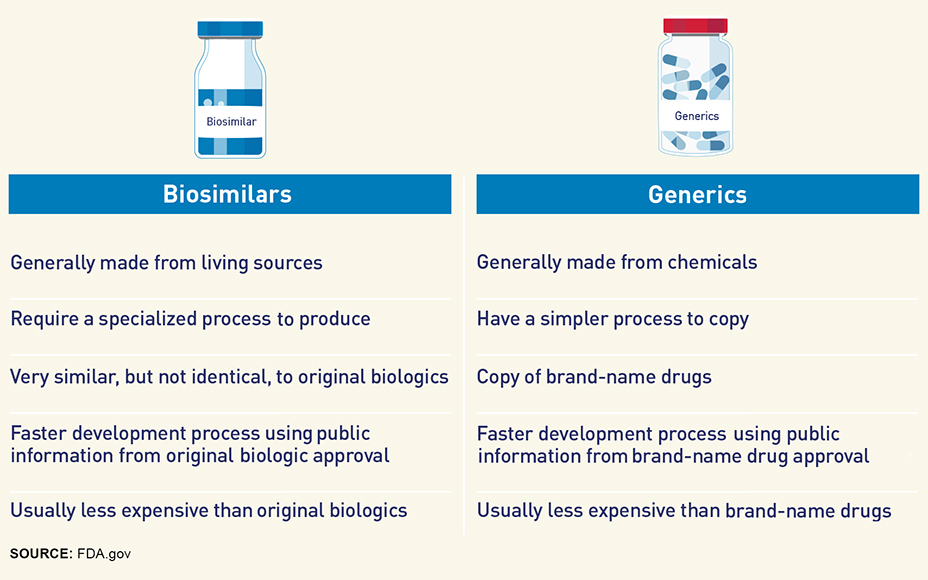
FDA Outlines Generic Safety and Efficacy - Policy and Medicine
5 (512) · € 28.50 · Auf Lager

Saunders Nursing Drug Handbook 2024: 9780443116070: Medicine & Health Science Books @

Exploring the Drug Development Process
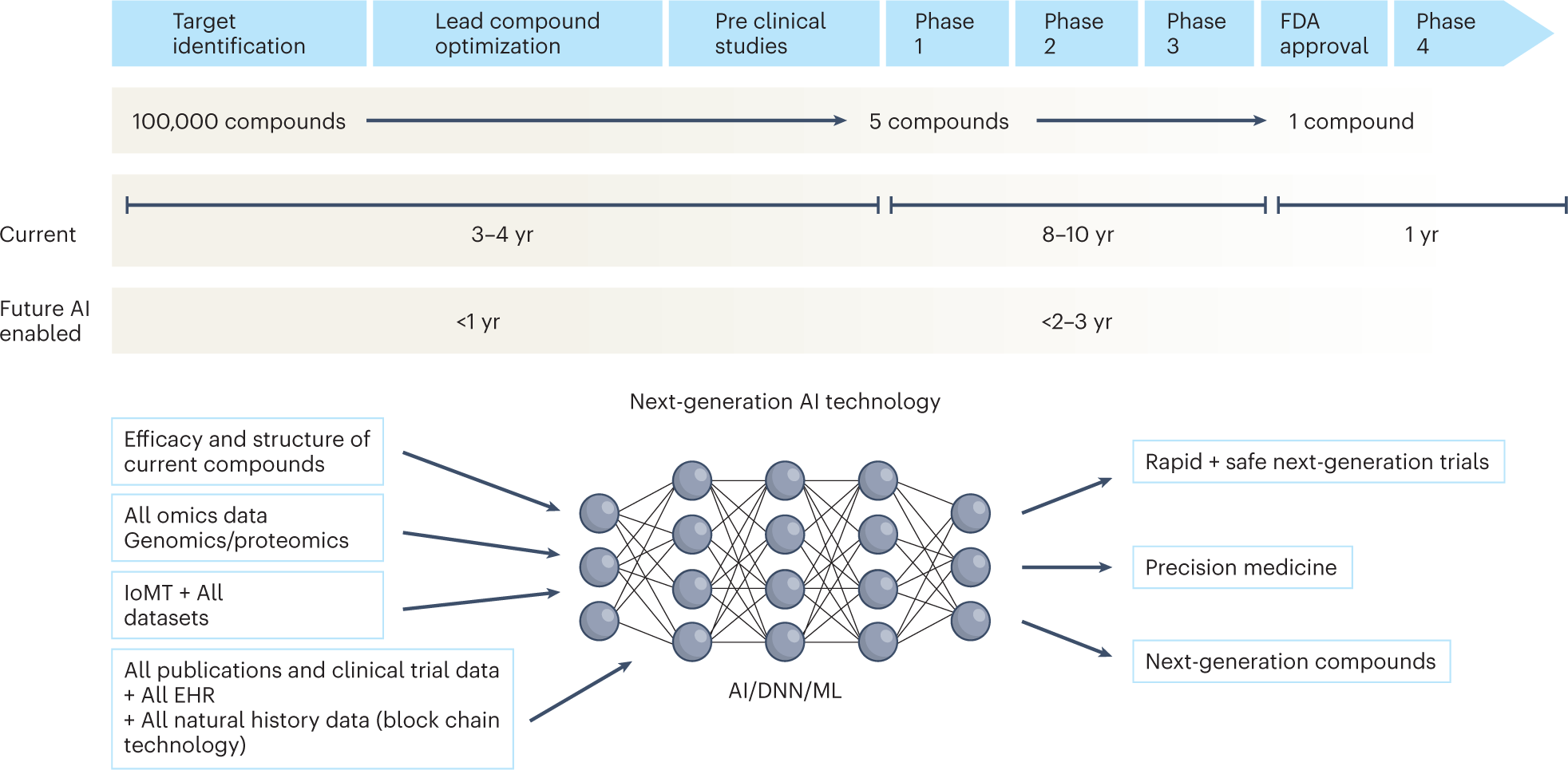
The next generation of evidence-based medicine

Emerging Strategies for Drug-Product Comparability and Process Validation: Part 2 - BioProcess InternationalBioProcess International

FDA Novel Drug Report for 2023 Cites 84% First-Cycle Approvals - What about Generic Drugs?
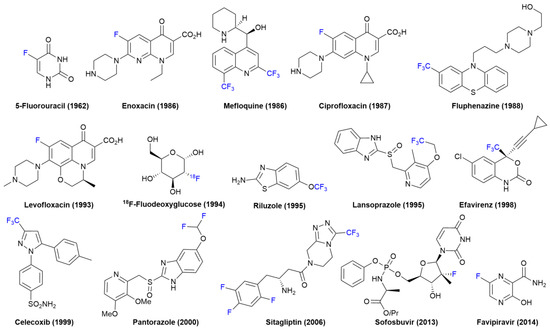
IJMS, Free Full-Text

Regulating and Authorizing Medicines: A Comparison of the FDA and EMA

Software as medical device clinical trials as per US FDA
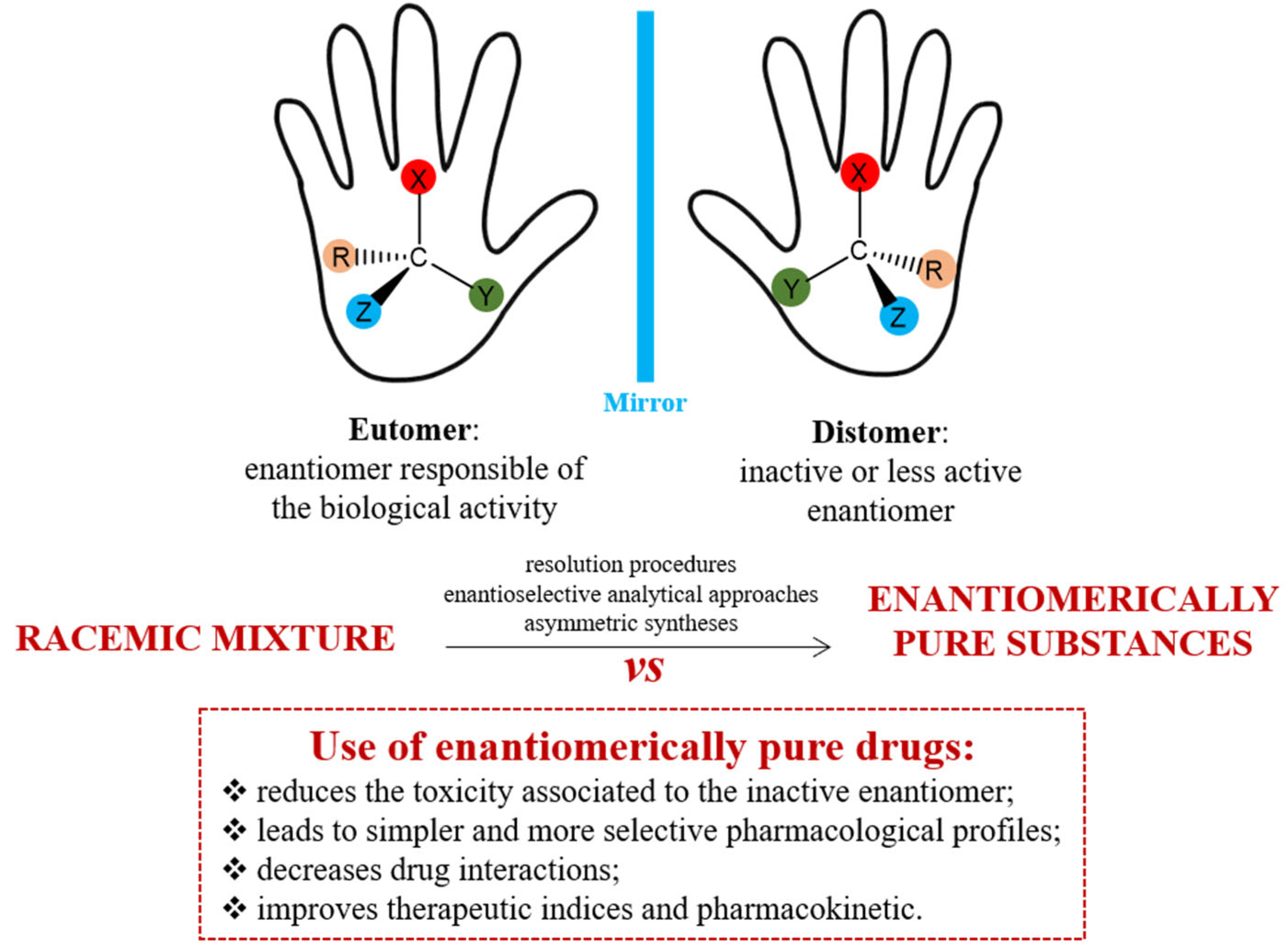
Applied Sciences, Free Full-Text
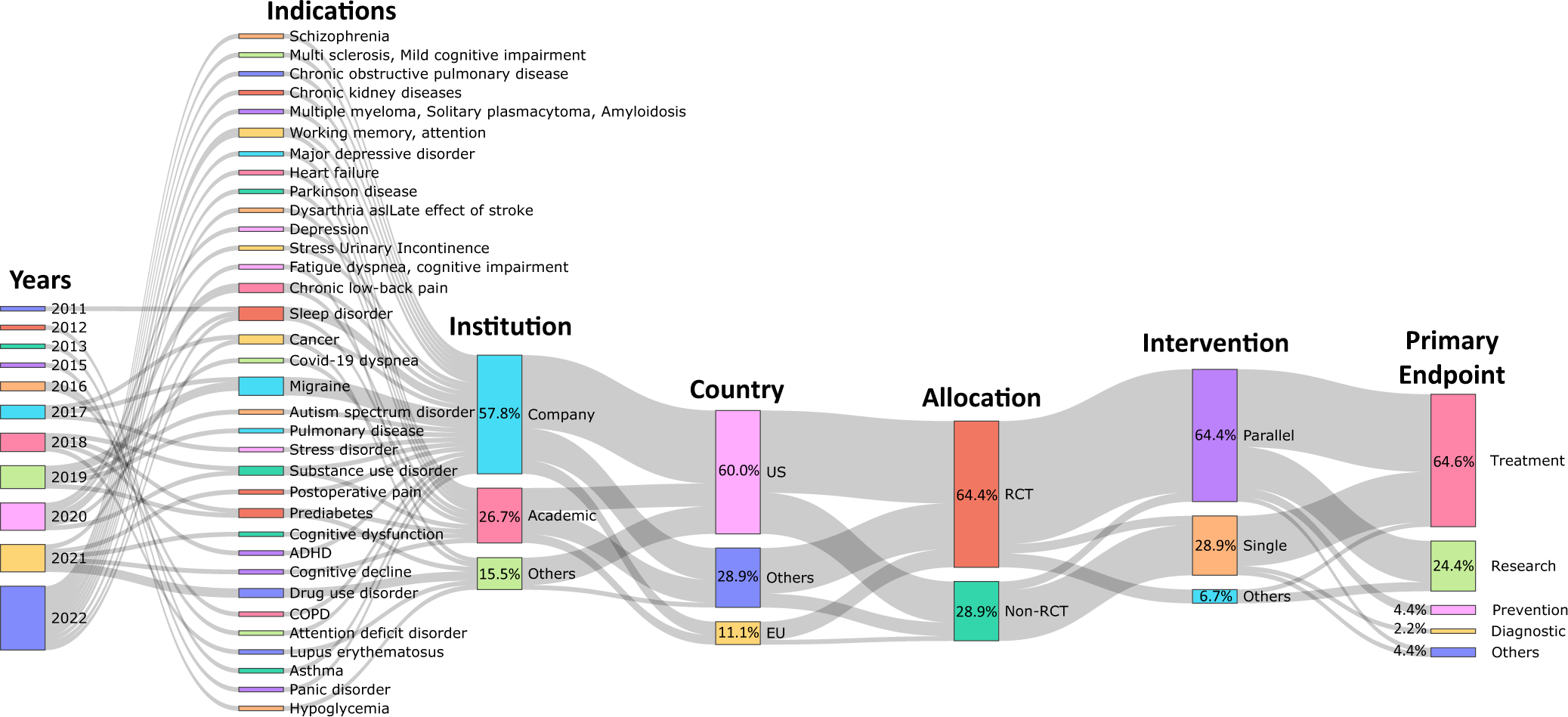
Digital therapeutics from bench to bedside
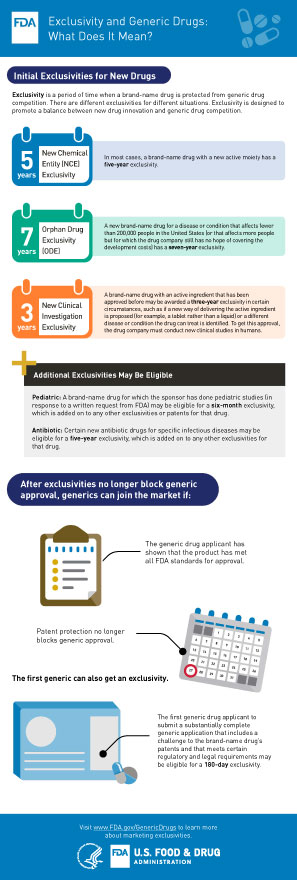
Frequently Asked Questions on Patents and Exclusivity

Mitigating the Inflation Reduction Act's Adverse Impacts on the Prescription Drug Market – USC Schaeffer

QbD IR Tablets - FDA Example
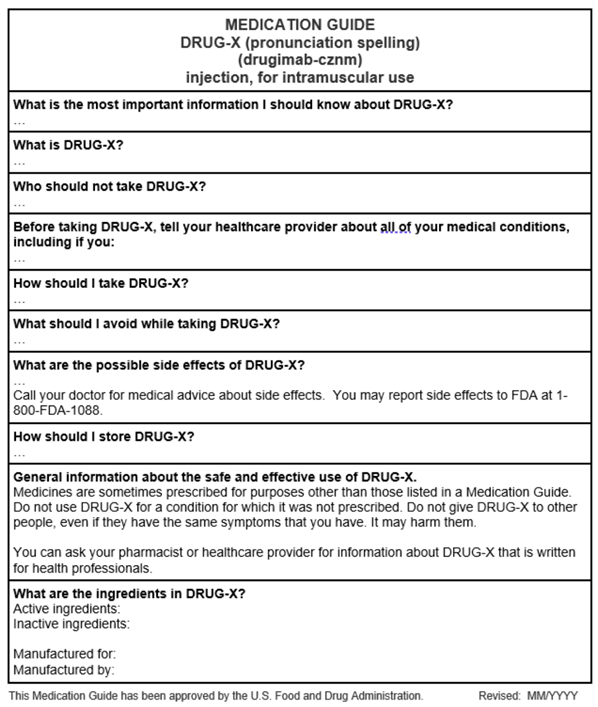
Patient Labeling Resources

Insight on 510(k) Clinical Studies: An In-depth Guide