cobas® SARS-CoV-2 & Influenza A/B Test
4.7 (608) · € 33.99 · Auf Lager

cobas® SARS-CoV-2 & Influenza A/B v2, Qualitative nucleic acid test for use on the cobas® 5800/6800/8800 Systems
Diagnostics, Free Full-Text
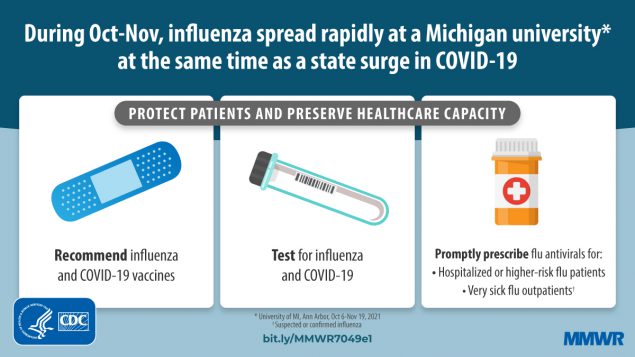
Influenza A(H3N2) Outbreak on a University Campus — Michigan, October–November 2021

Evaluating the Ability to ID (COVID-19) NOW: a Large Real-World Prospective Evaluation of the Abbott ID NOW COVID-19 Assay

SARS-CoV-2 detection using reverse transcription strand invasion based amplification and a portable compact size instrument
Roche Granted FDA Emergency Use Authorization for Cobas SARS-CoV-2 & Influenza A/B Test - COVID-19 - mobile.
TaqPath COVID-19 Multiplex Diagnostic Solution

Performance Evaluation of the Microfluidic Antigen LumiraDx SARS-CoV-2 and Flu A/B Test in Diagnosing COVID-19 and Influenza in Patients with Respiratory Symptoms

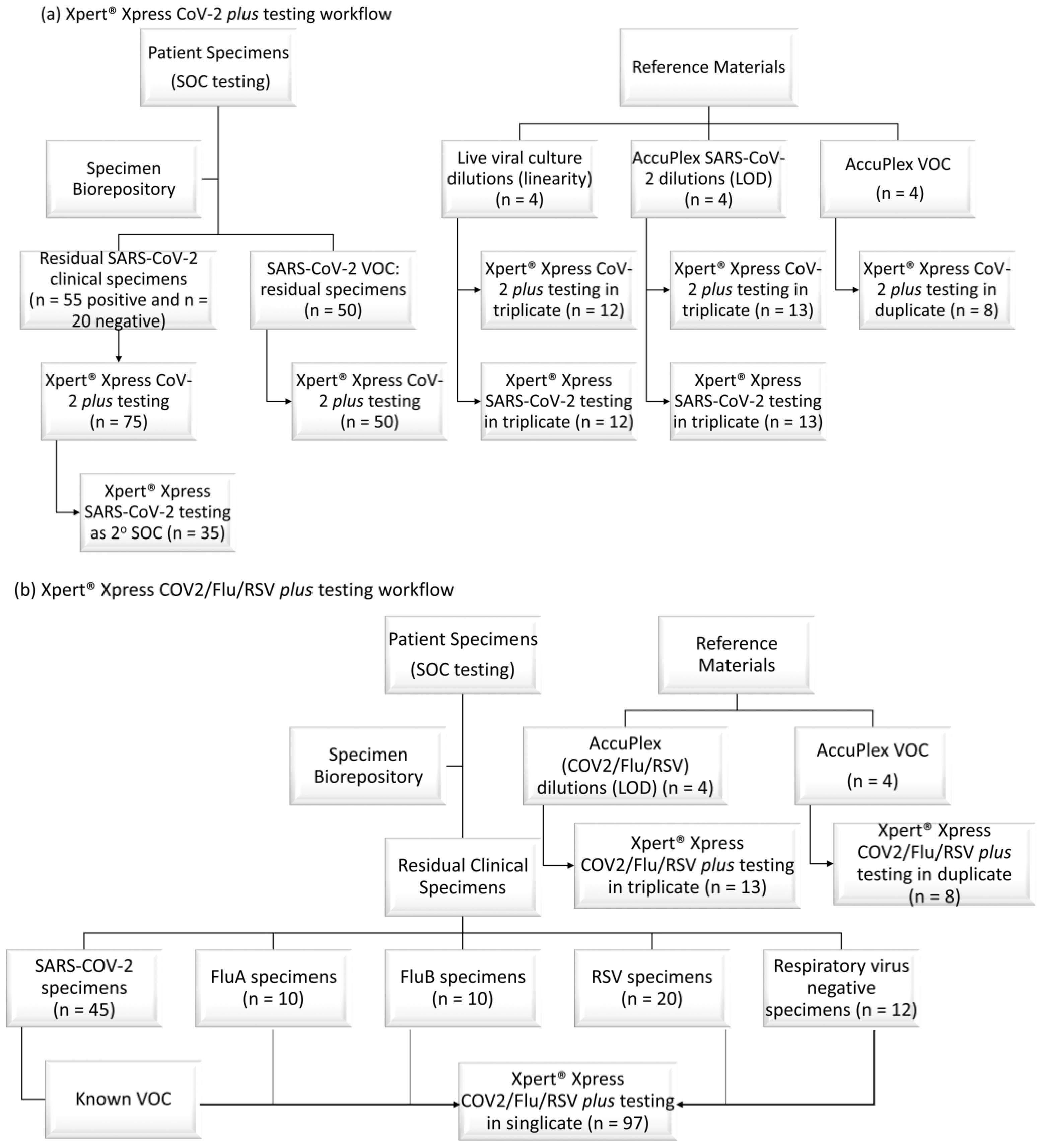
Clinical evaluation of the GeneXpert® Xpert® Xpress SARS-CoV-2/Flu/RSV PLUS combination test

Two Years into the COVID-19 Pandemic: Lessons Learned

FDA authorized molecular point-of-care SARS-CoV-2 tests: A critical review on principles, systems and clinical performances - ScienceDirect
SARS-CoV-2 & Flu A/B Rapid Antigen Test
STATUS™ COVID-19/Flu A&B Rapid Antigen Test - Aurora Biomed
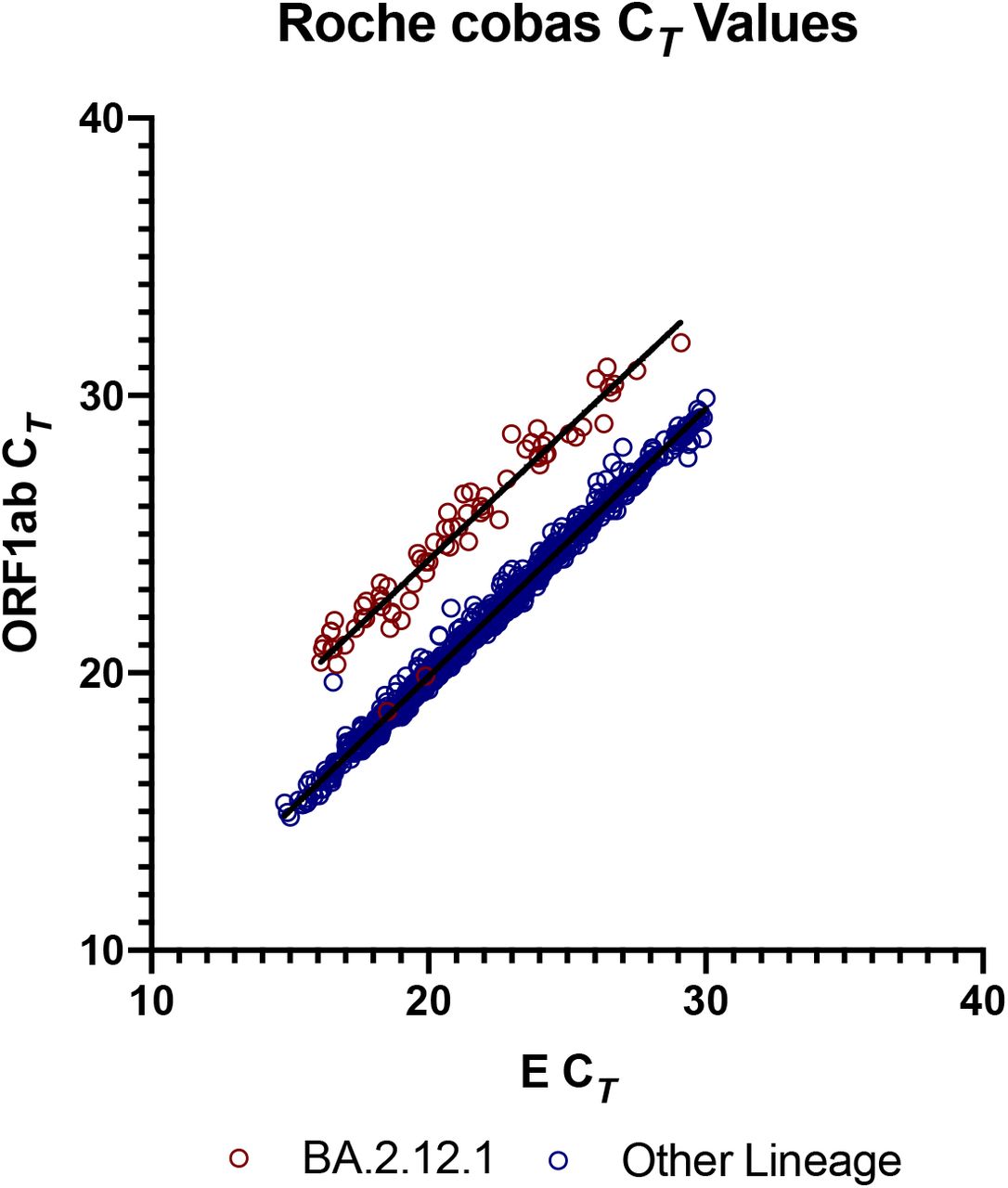
Partial ORF1ab Gene Target Failure with Omicron BA.2.12.1