Catalog of Regulatory Science Tools to Help Assess New Medical
4.6 (247) · € 22.00 · Auf Lager

What Is Risk Management in Healthcare?
The imperative for regulatory oversight of large language models (or generative AI) in healthcare

CDRH Regulatory Science Priorities

Regulatory Considerations on the use of Machine Learning based tools in Clinical Trials

Regulatory Science Tools Reduce Risk in New Medical Devices

CODE-EHR best-practice framework for the use of structured electronic health-care records in clinical research - The Lancet Digital Health
Accelerating med tech: FDA catalog of research tools showcases agency's commitment to innovators

Pilot III - Presentation 'Regulatory Support to Spanish Academia from STARS Core to Comprehensive Curriculum' - Stars

FDA's Catalog of Regulatory Science Tools - EMMA International

Making Sense of Your Self-Assessment, Science

Regulatory Science – An Underappreciated Component of Translational Research: Trends in Pharmacological Sciences

USA Overview of the Medical Device Market (video)

O'Leary's Blog - Regulatory considerations for digital surrogate endpoint response biomarkers

The evolving regulatory landscape in regenerative medicine - ScienceDirect
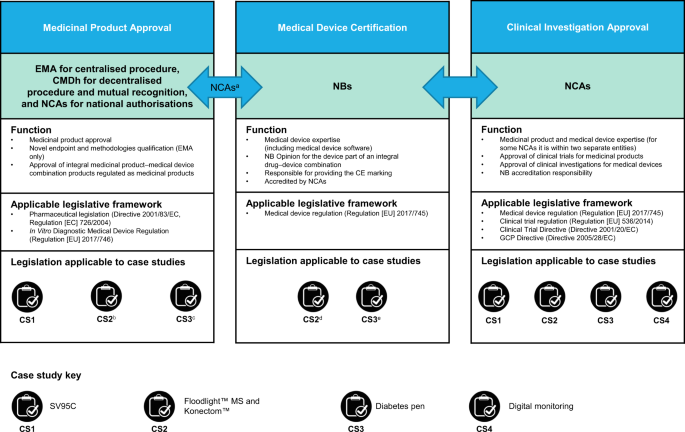
Evolving regulatory perspectives on digital health technologies for medicinal product development