- Startseite
- anti-rauch-reiniger
- The uptake of tau amyloid fibrils is facilitated by the cellular prion protein and hampers prion propagation in cultured cells - De Cecco - 2020 - Journal of Neurochemistry - Wiley Online Library
The uptake of tau amyloid fibrils is facilitated by the cellular prion protein and hampers prion propagation in cultured cells - De Cecco - 2020 - Journal of Neurochemistry - Wiley Online Library
4.8 (189) · € 22.99 · Auf Lager

PDF) Lymphocyte‐Activation Gene 3 Facilitates Pathological Tau Neuron‐to‐Neuron Transmission

Mutant Presenilin 1 Dysregulates Exosomal Proteome Cargo Produced by Human-Induced Pluripotent Stem Cell Neurons

Delineating the Role of GxxxG Motif in Amyloidogenesis: A New Perspective in Targeting Amyloid-Beta Mediated AD Pathogenesis

Potential Role of Natural Polyphenols against Protein Aggregation Toxicity: In Vitro, In Vivo, and Clinical Studies

Cryo-EM structure of RNA-induced tau fibrils reveals a small C-terminal core that may nucleate fibril formation

Anchorless risk or released benefit? An updated view on the ADAM10-mediated shedding of the prion protein

Anchorless risk or released benefit? An updated view on the ADAM10-mediated shedding of the prion protein

Major Differences between the Self-Assembly and Seeding Behavior of Heparin-Induced and in Vitro Phosphorylated Tau and Their Modulation by Potential Inhibitors

Prion-Like Propagation of Post-Translationally Modified Tau in Alzheimer's Disease: A Hypothesis
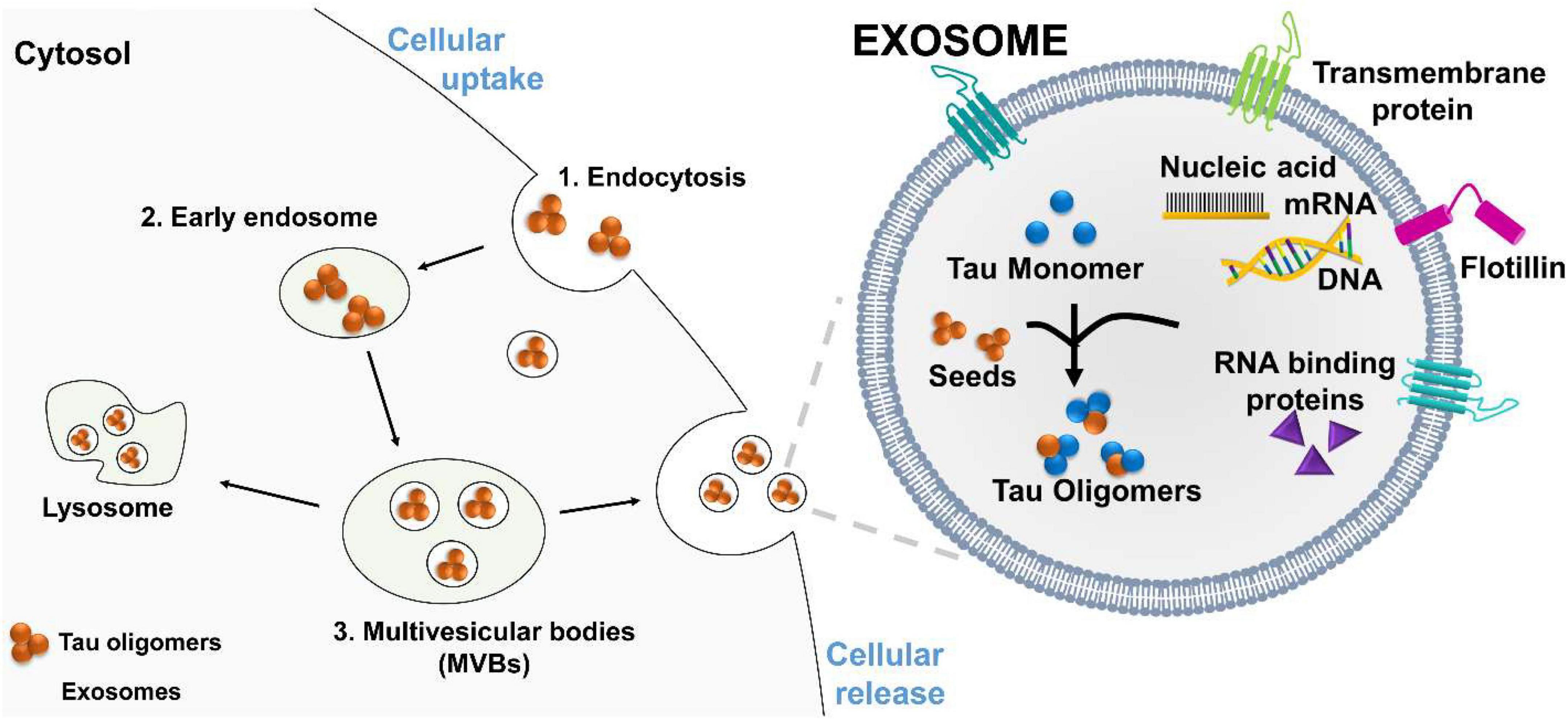
Frontiers The prion-like transmission of tau oligomers via exosomes

Interactions between SARS-CoV-2 N-Protein and α-Synuclein Accelerate Amyloid Formation

The Role of Heme and Copper in Alzheimer's Disease and Type 2 Diabetes Mellitus

PDF) Lymphocyte‐Activation Gene 3 Facilitates Pathological Tau Neuron‐to‐Neuron Transmission

Current Progress in the Development of Probes for Targeting α-Synuclein Aggregates

Major Differences between the Self-Assembly and Seeding Behavior of Heparin-Induced and in Vitro Phosphorylated Tau and Their Modulation by Potential Inhibitors












